COVID-19
SAB-185: Advancing Novel Polyclonal Therapeutic for COVID-19
Founded in 2014, SAB Biotherapeutics is dedicated to the development and therapeutic application of our novel technology that harnesses the native human immune response to disease and rapidly translates it to a targeted, safe, high-potency immunotherapy on a large scale.
A global pandemic, caused by a fast-spreading, airborne infectious disease like COVID-19, has highlighted the need for effective therapeutics that can be quickly scaled up and deployed, a unique challenge our technology is modeled to address. The speed and sophistication of our platform – that leverages genetically-engineered cattle to produce fully human antibodies – enables us to address a global health threat.
The right therapeutic technology, at the right time
-

SAB-185, a COVID-19 therapeutic, represents a highly-differentiated treatment option that provides a highly-specific match against the complexity, diversity, and the mutations SARS-CoV-2 presents.
-
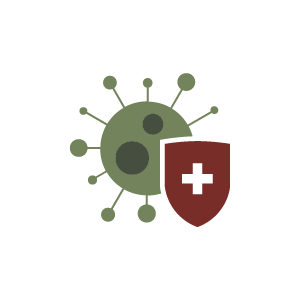
Our novel human polyclonal antibody candidate for COVID-19 may have value both therapeutically, as a treatment for patients infected with the virus, and for immediate protection (passive immunity) when a vaccine is not an option. Designed to be produced without the need for human convalescent plasma, blood donations, or serum, SAB-185 production efficiently to produce large volumes of fully human polyclonal antibodies targeted specifically to SARS-CoV-2.
Polyclonal Antibodies: Broad Spectrum Effectiveness
SAB’s technology platform leverages the natural human immune response to develop next-generation, fully human polyclonal antibody therapeutics without the need for human serum, extending both the safety and potency. These highly efficacious antibodies have proven to neutralize and block disease, and also address future mutations. SAB’s platform represents, for the first time, the ability to produce targeted, fully human, high potency polyclonal therapies on a commercial scale.
SAB-185: A highly-potent human polyclonal antibody therapeutic targeting SARS-CoV-2
- Highly-differentiated, innovative approach to infectious disease developed and optimized over more than a decade
- Advancing in collaboration with USG COVID-19 Response (formerly Operation Warp Speed) as part of the ACTIV-2 Adaptive Platform Treatment Trial
- Recently dosed first patient in NIH NIAID-sponsored and funded Phase 2/3 Adaptive Trial
- Completed enrollment of Phase 1 study in healthy volunteers and a Phase 1b trial in ambulatory COVID-19 patients
Multi-faceted Therapeutic Developed to Address Virus Mutation
- Demonstrated high neutralization potency against mutants in circulating strains
- Demonstrated neutralization of monoclonal escape mutants
A Model for Future Response
Four awards, totaling $129.5 million dedicated to SAB’s rapid response capability and COVID-19 therapeutic candidate, SAB-185, announced since the pandemic began. These include:
- $57.5M from the Biomedical Advanced Research Development Authority (BARDA), part of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services for COVID-19 therapeutic manufacturing
- $35.6M awarded from U.S. Department of Defense’s (DoD) Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND) on behalf of the Office of the Assistant Secretary of Defense for Health Affairs (OASD(HA)) and the Defense Health Agency (DHA) for advancement of SAB-185 and Scaling of Rapid Response Antibody Program
- $9.4M for the development of SAB-185 through preclinical studies, supported by BARDA
- $27 million progressive and competitive, three-stage, multi-year contract from the U.S. Department of Defense to develop a novel Rapid Response Antibody Program
- State-of-the-art, pharmaceutical platform technology capable of rapidly and reliably producing antibody-based Medical Countermeasures (MCM) for biological threats
- Program goal to accelerate delivery of potent, human, polyclonal antibody therapeutics to address known, and novel, emerging biodefense (viral, bacterial or toxin) threats
- Awarded through DoD’s Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense (JPEO-CBRND) Joint Project Lead CBRND Enabling Biotechnologies (JPL-CBRND-EB).
Rapid Response Medical Countermeasure
SAB’s approach has expedited the rapid development of a therapeutic for COVID-19, with the ability to move from a target to a therapeutic candidate in as few as 90 days. This “on the ready” therapeutic engine, provides a latest generation platform capability to address a novel or emerging disease deploying the same process with a new immunogen to create a new therapeutic.
“We are continually exploring new technologies to ensure the security and safety of our armed forces as well as the American and global public. SAB Biotherapeutics’ unique antibody platform shows real potential to address the critical need for fighting coronavirus and establishing a truly responsive model for combatting future threats.”
—MATTHEW HEPBURN, M.D., JOINT PROJECT LEAD CBRN DEFENSE ENABLING BIOTECHNOLOGIES
“SAB Biotherapeutics’ novel immunotherapy platform provides a new and innovative solution to rapidly respond without the need for human plasma. For future pandemics, SAB’s platform may allow us to even more rapidly respond to patients’ needs.”
—BILL MEZZANOTTE, M.D., EXECUTIVE VICE PRESIDENT AND HEAD OF R&D–CSL BEHRING
A modernized approach to convalescent plasma & the future of pandemic response
SAB’s DiversitAb™ platform has a greater capability to target and scale than other human polyclonal antibody programs. Our platform can consistently and reliably produce fully human antibodies without the need for convalescent plasma from human donors. Tc Bovine–our genetically-engineered cattle–mount the same immune response as survivors of a disease, such as COVID-19, with a much higher concentration of targeted neutralizing antibodies. In addition eliminating the need to identify, screen, and draw blood from recovering volunteers, our novel approach opens the door to medicines that are potentially more potent, safer, and longer-lasting than current antibody therapies.
-
POTENCY
As a ruminants, cows inherently have a more robust immune response than humans. This response is stimulated when an immunogen specifically and exclusively targeted to a virus, such as SARS-CoV-2, is introduced, activating the entire disease fighting force of human antibodies. We further boost the response with a specialized vaccine formulation and strategy that maintains a high level of antibody production over an extended period of time and targets plasma collection at the highest levels. These optimized technologies result in large amounts of diverse, high-titer, high-avidity antibodies resulting in a high-potency therapy.
-
SCALE
As a large animal production system, greater volumes of neutralizing antibodies are generated that can be collected up to three times per month per animal. Production can be ramped up by simply continuing to immunize and collect plasma, and by adding more animals to the effort.
-

SAFETY & CONSISTENCY
Unlike human plasma donors, who may have previous exposures to many pathogens and varying genetic backgrounds, SAB’s platform offers a large scale, consistently managed donor pool, genetically representing a single human donor. In addition, because our Tc Bovine are injected with a non-infectious subunit, they produce antibodies to the immunogen without becoming infected or getting sick.

"SAB's novel immunotherapy platform, the DiversitAb TM enables a scalable and reliable production of targeted, higher potency neutralizing antibody product than has been previously possible."
—EDDIE J. SULLIVAN, PHD, PRESIDENT, CEO & CO-FOUNDER, SAB BIOTHERAPEUTICS
A novel approach using nature’s genius to fight disease
Our therapies leverage the native human immune response, providing a highly specific match against the complexity, diversity and mutation of disease.
SAB’s streamlined proprietary development process eliminates the selection step needed to select the most potent antibody candidates. Rather, a specifically designed immunization for genetically engineered cattle activates the entire disease fighting force of fully human antibodies. Hyperimmunization of the cattle results in diverse, high-titer, high-avidity antibodies that are then captured in the large amounts of plasma resulting in a high-potency therapy.
Tc Bovine-produced human antibodies have shown to “behave” identically to those produced in humans. As such, our therapeutic works with a patient’s own immune system to both neutralize a virus and activate the other components of the immune system that are important in helping the body fight disease. SAB data from across our clinical platform has shown that our antibodies are effective even when antivirals or monoclonal antibodies have become ineffective due to virus or pathogen mutation. This is one of the compelling reasons why polyclonal antibodies have the potential to serve as the primary line of defense against mutating viral targets like COVID-19.
Competence with coronaviruses
SAB has proven experience that has prepared us to address the current COVID-19 threat and future threats. Since 2014, we have produced more than a dozen antibodies to combat infectious diseases, including Zika, Ebola and Middle East Respiratory Syndrome (MERS), a coronavirus.
-
SAB-301, a treatment for MERS-CoV, is one of the most advanced therapeutics against a coronavirus. It has completed a Phase I clinical safety trial in healthy volunteers and is progressing to a Phase 2/3 trial in the Middle East–both sponsored by NIH NIAID and with support from BARDA.
-

More than a decade ago, we developed human antibodies to the past Severe Acute Respiratory Syndrome, or SARS-1, a precursor to the virus causing COVID-19, SARS-CoV-2. Our tremendous amount of historical information and data from working with coronaviruses is informing how we target antibodies to this new coronavirus.
-

An SAB therapeutic has demonstrated safety and efficacy in a successful first-in-human clinical trial for Antibiotic Resistant Mycoplasma, sponsored by Brigham and Women’s Hospital and Harvard Medical School.
Our obligation + responsibility
While we’re leveraging our unique capabilities to rapidly respond to this public health emergency, we’re also doing our part to limit the continued spread of the virus. Our leadership is working closely with national, state and local healthcare officials to implement appropriate precautions while continuing our critical work on a treatment for COVID-19.
OUR TEAM
The health and safety of our team is the top priority. We’ve taken precautionary measures to limit the potential exposure of our team and are taking additional precautions to protect our essential laboratory and manufacturing colleagues whose jobs require they be on site.
OUR BUSINESS
SAB continues to closely monitor the pandemic and take proactive measures to ensure business continuity, particularly given the critical ongoing operations as we work to provide a solution for COVID-19.
OUR FACILITIES
We are working closely with Sanford Research, implementing policies and limiting visitors to other SAB facilities.
OUR INDUSTRY
SAB Biotherapeutics has been an active member of the Biotechnology Innovation Organization and is working closely with the rest of the industry to meet the challenge of the COVID-19 pandemic.
Read More
We want to remind you that SAB does not work directly with live target viruses as part of our development process. Our team works with non-infectious subunits of the virus in developing our therapeutics.
It’s global. And it’s personal.
At SAB, this pandemic allows us to deliver on the promise of our technology: to make a positive impact on human health with our novel, breakthrough technology. COVID-19 is a global problem that has quickly become very personal to all of us.
And it will take all of us working together, using all of our innovations and technologies, to address the current pandemic and to be ready to alleviate the next threat.
FOLLOW THE LATEST COVID-19 UPDATES:
-
Follow our Twitter feed @SABBantibody for the latest news and updates.
-
Visit the CDC website for more information on the COVID-19 pandemic.
